The electrolytes in advanced batteries are based on incredibly complicated formulations. If you calculated all of the possible combinations of solvents, salts and additives while trying to find the optimal ratios for just one electrolyte formulation, it would add up to an extraordinary number of possibilities.
Because it plays such a critical role in battery design, the science of electrolytes continues to advance at an encouraging clip. Charged recently chatted with Dr. Dee Strand – our go-to battery tech expert at Wildcat Discovery Technologies – to learn more about the complicated mixtures that transport those little lithium ions.
Wildcat is a very active research group at the forefront of the race to find better battery materials. Its high-throughput combinatorial chemistry techniques can build and test prototype batteries up to 100 times faster than a standard lab – a process that’s ideally suited for optimizing materials with seemingly endless formulation possibilities.
“Commercial electrolytes have a lot of components,†explains Strand. “Depending on the application, they consist of at least two solvents (and usually more co-solvents), a salt (or multiple salts), and a package of about five or six different additives.â€
Solvents
Electrolyte formulations start with a solvent that has a high dielectric constant to help dissolve the salt. Often these high-dielectric-constant solvents have a high viscosity, so they are blended with a low-viscosity solvent. Then, other co-solvents are added to help improve various properties like low-temperature performance or power performance.
Salts
Lithium salts are dissolved in the electrolyte solvents. Solvent molecules surround the lithium ions as they move back and forth between the cathode and the anode. The salt’s anions can play an important role in how strongly it binds to the lithium – ideally you want it to be totally dissociated.
Additives
There are many different categories of additives that are designed to do all sorts of things. Some common additives, like vinylene carbonate, help the solid electrolyte interface (SEI) layer form on the anode. Another category of additive is a redox shuttle, which creates an intrinsic overcharge protection mechanism that improves safety.
“When developing an electrolyte, you want to start with fairly simple formulations,†explains Strand. “Say, for example, you take a certain solvent and one additive and test to see how that performs. Then you add another additive and see what that does. Perhaps Additive A helps the battery at high temperatures, Additive B helps at high voltage, and Additive C does something else. You really want to map out the effects of each component. When you start combining them you see that some are synergistic, but more often they’re antagonistic. Two additives may show improvements on their respective metrics when tested separately, but put them together and they’ll actually hurt each other’s performance. So you’re looking for those cases where the performance of A and B together is greater than the sum of A and B separately.â€
More energy, more problems
The battery industry, in general, is looking for higher energy density, whether for EV applications or to make our cell phones last longer. A lot of high-energy-density research is focused on cathodes that have higher capacities or that can be charged to higher voltages to improve overall energy.
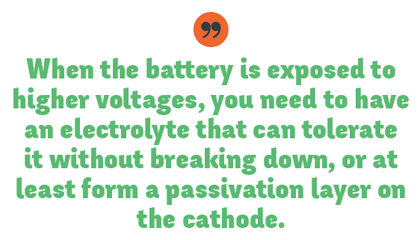
“Many high-energy cathodes, however, have a high nickel content which is very reactive with the electrolyte,†explains Strand. “Also, when the battery is exposed to higher voltages, you need to have an electrolyte that can tolerate it without breaking down, or at least form a passivation layer on the cathode. That layer also helps you at high temperatures where all of those problems – from electrolyte oxidation to its reactivity with the nickel – become much worse. So it’s critical to stabilize that cathode surface with electrolyte additives in order to get high cycle life at high voltages and high temperatures.â€
High-nickel cathodes also tend to generate a lot of gas. Gases can lead to safety issues and are often related to the formation of high-impedance or high-resistance layers that reduce power performance. “As you go to these more reactive and higher-voltage cathode materials, it really affects all performance aspects of the battery,†says Strand. “So, finding better electrolyte formulations becomes very important to minimize those effects.â€
Another growing application for lithium-ion batteries is start-stop vehicles, in which energy density is less important, but you need high cycle life and the ability to supply high power over a wide temperature range. The battery needs to be able to operate from a cold stop, but it also tends to be near the engine – under the hood – so it must tolerate high temperatures.
Strand explains that for those electrolyte formulations, you need to be very mindful of keeping low-viscosity formulations and low-impedance SEI layers. You also need to stabilize the surface of the electrodes. So, the electrolyte can play a role at both sides of the temperature spectrum, and that’s due to solvents and additives.
Solid electrolytes
Another promising area of research for increasing energy density is solid electrolytes. This technology has the potential to allow the use of lithium metal for an anode, as opposed to graphite. The benefit is that the entire anode is active material with a much higher specific capacity. One of the challenges with lithium metal is that as you charge and discharge, the lithium ions tend to dissolve and redeposit in the form of dendrites – long growths that resemble needles. Eventually the dendrites can puncture the separator and short the battery. It also results in a high surface area of lithium, which is very reactive.
The promise of using a stiff solid electrolyte is that it can prevent those dendrites from growing simply as a mechanical barrier. There are also safety benefits, because instead of the flammable and volatile liquid organic electrolytes, you could have an inorganic solid. So, if the battery ever finds itself in a very hot environment, you wouldn’t have to worry about the build-up of high vapor pressure.

“One of the problems with solid electrolytes is that it’s harder to move lithium through them compared to liquids,†explains Strand. “That being said, there have been really significant advances in material discovery for solid ceramics that can move lithium almost as well as liquids. The challenge is integrating them into a battery. They are typically hard, brittle ceramic materials that are difficult to manufacture in thin sheets and put into the cell assembly without breaking.â€
Solid-state batteries have a lithium metal anode, a ceramic membrane that conducts lithium (you no longer need the plastic separator) and then the cathode. So the membrane needs to be thin, about the thickness of a separator, otherwise you’re losing space in your battery to something that’s not active material.
“Finally, the last challenge to creating commercial solid-state batteries is in the cathode,†says Strand. “Today’s cathodes are a bunch of solid active materials held together with binder and pores in between the material. Those pores are important because it allows the liquid electrolyte to infiltrate and move lithium in and out of the cathode. So once you find a great solid electrolyte separator membrane that conducts lithium ions well, what do you do about your cathode? Without a liquid to fill in the pores, how do you access the active material throughout the whole cathode?â€
One of Wildcat’s many research projects involves a combination of ceramics and polymers, both lithium ion conducting materials that could be put into the cathode’s pores and also be used as a separator electrolyte. “The polymer gives you better mechanical properties in terms of flexibility,†says Strand, “and the ability to laminate layers together so that you can help reduce that interfacial impedance between layers. The challenge with these types of materials is getting high ionic conductivity, because the polymer tends not to be great. That’s the direction of our research.â€
Semi-solid electrolytes
Some companies have been working on gel-like electrolytes, often referred to as semi-solid. Essentially, they add solvents to a polymer, like polyethylene oxide (PEO), to vastly improve the lithium ion conductivity. The solvents swell the polymer, and a lot of the ion transport then occurs in the solvent. The technique can improve the safety aspects of a battery because the solvent is trapped in a gel. Semi-solid electrolytes do not work well with lithium metal anodes, because the swollen polymer gel is too soft to prevent the dendrite growth. However, they can be used with traditional electrode materials.

Testing is tricky
On top of all of the possible combinations of solvents, salts and additives, there are also challenges in testing sample cells with new electrolytes. It is very difficult to open up a battery and collect samples to test the chemical makeup of an electrolyte or the SEI layer. “As soon as you open up the battery, things change,†explains Strand. “A lot of the techniques that exist to study the SEI layer are under vacuum. This will evaporate any liquids and the SEI is kind of a fluid layer. These analytic techniques themselves are a great area of science, but one could spend a PhD thesis studying the SEI for a single electrolyte formulation, then you put in another additive and it changes it.â€
“So, we look at the effects of new formulations. Our methods probe the properties of the SEI in an indirect fashion based on their effect on the cell performance. When additives react with either the cathode or the anode, the layers that form tend to increase the impedance of the battery. We look for additives that keep the impedance low. But, on the other extreme, if you did not form any layer there would be zero impedance, which is very bad for high-temperature performance because there is nothing to stop the reactivity. So we want a balance of low impedance and high thermal stability for the battery. This indicates that the battery most likely has a thin uniform layer with good ionic conductivity to protect that surface.â€

Combine, test and repeat – the Wildcat edge
Every component that makes up a commercial electrolyte is included for a very specific reason. So the scientists striving to develop better formulations for the next generation of batteries spend their time determining what role a new material could play in the compositional space. This requires many experiments to map out characteristics, try different combinations and look for those synergies that get the optimal performance out of the electrolyte.
This type of work is what Wildcat prides itself on. “We can formulate lots of electrolyte variations and make batteries with enough replicates to get good statistical results,†explained Strand. “Traditional electrolyte companies have a portfolio of additives that they will mix and match – it’s usually a pretty slow process, and they may only try a few different concentrations. Because of our high-throughput techniques, we can try dozens. They may try two ratios of solvents, for example, where we could try 20, then map out a phase diagram to compare them. The experimental rounds for these tests – depending on the C-rate used for cycling – might be as long as four to six weeks before you have results. So wouldn’t you rather do 1,000 experiments on different formulations than a few dozen?â€
Wildcat also has a large additive discovery program looking for new molecules to offer to the industry. There are other companies that do this, but Wildcat says its proprietary techniques give it the bandwidth needed to greatly increase the probability of finding the optimal formulation to fix a problem, without messing anything else up.
A few of Wildcat’s recent electrolyte-related discoveries (that it can discuss with us), include:
SuperFilm additive
A new modular additive concept, which Wildcat calls SuperFilm, is used to bind to the molecular core of other additives, enabling uniform deposition on the electrode surface. The company says that attaching conventional additives to the core molecules provides improved SEI stability, resulting in increased coulombic efficiency, cycle life, and thermal stability.
“For example, perhaps you want a nice uniform passivation layer to help you at high voltage, but you don’t want to add too much impedance to your cell,†says Strand. “Our SuperFilm additives are very helpful at stabilizing those very high-energy high-nickel containing materials, like NCA and NCM. Also, by decoupling the requirements for uniform coating and chemical stability, new classes of additives can be used, so there is a lot of room for exploration in this area.â€
Wide temperature-range electrolytes
Wildcat reports quite a few innovations that could encourage the use of Li-ion batteries in the quickly growing stop-start market. The company has developed electrolyte formulations that improve power performance at low temperature and improve or maintain the high-temperature cycle life relative to baseline electrolyte formulation for both NMC/graphite and NMC/LTO electrode chemistries.
Novel non-carbonate electrolyte formulations for silicon anodes
Many researchers in the battery industry are looking for ways to replace the graphite anode with silicon. Silicon is widely considered to be the next big thing in anode technology, because it has a theoretical charge capacity ten times higher than that of typical graphite anodes. This allows you to use less anode material and fill up that extra space with more cathode material – effectively increasing the overall energy that is contained within the same volume.
The problem with the current state of silicon anodes is that repeated expansion and contraction during charging and discharging leads to drastically reduced cycle life, due to two big failure mechanisms: problems with the electrode itself and cracking of the SEI layer.

One solution to the SEI layer problem might be found in a DOE-funded project that Wildcat has underway to develop better electrolytes and additives for silicon anodes. Wildcat says the project, named EM4, has been very successful at making more mechanically and electrochemically robust SEI layers on the anode. So as volume changes occur, the SEI layer doesn’t crack. “We’re now getting much longer cycles on the particular silicon anode materials that we’re using,†says Strand. “It’s very exciting.â€
Gas-reducing efforts
Gas generation is especially problematic as cell markers push toward increased voltages. Wildcat has identified a lot of additives and formulations that have reduced gas significantly even at voltages of 4.9 V, like the nickel-manganese spinel chemistry, which is a very severe environment for an organic electrolyte.
To increase the speed of gas-reducing discoveries, Wildcat developed gas-sensing channels that can simultaneously measure gases released by cells, along with the normal electrochemical measurements of capacity, coulombic efficiency and cycle life. This allows for precise detection of gassing directly inside the cell. The new method enables continuous gas measurements while cells cycle.
When coupled with the company’s other techniques, this approach lets its speedy scientists rapidly evaluate gas evolution for thousands of electrolytes in full cells, dramatically accelerating the development of improved additives and electrolyte formulations. With such a tool, it’s possible to start discovering very interesting correlations. For example, if certain materials have poor coulombic efficiency, do they also have a lot of gas generation? Or if materials have poor high-temperature cycle life, is that accompanied by a particular gas being released?
Science for hire
Wildcat’s high-speed discovery techniques are so unique that over 60 companies have turned to it for help since its inception in late 2006. In addition to working on many problems brought to them by battery companies from around the world, Wildcat is licensing several of their recent internal discoveries and actively seeking new electrolyte research collaborations.
Source: ChargedEVs


