Nanostructured electrodes in Li-ion batteries have several advantages, including shorter distances for charge-carrying particles to travel and a high surface area that provides more active sites for electrochemical reactions to occur. However, working with nanoscale materials is tricky – due to their tiny size, they can be hard to control and hold in place.
To address the challenges of nanoscale manufacturing, researchers at the University of Maryland, Baltimore County (UMBC) have turned to biological molecules called peptides. Peptides, which are made up of amino acids, bind to many different types of organic and inorganic materials. The research team has isolated a peptide that binds to lithium manganese nickel oxide (LMNO), a promising cathode material.
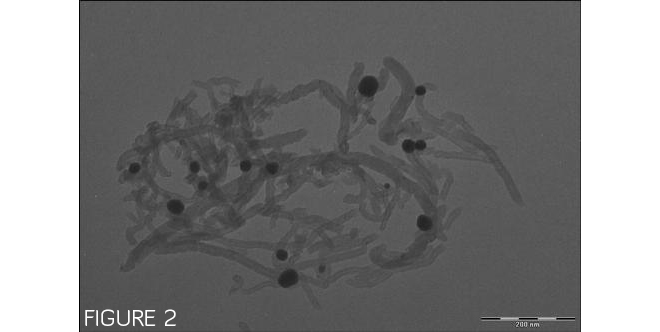
Figure 1 shows lithium manganese nickel oxide and carbon nanotubes clumping separately, with no specific interactions. However, when a multifunctional binding peptide is added to the mixture, as shown in Figure 2, the peptide binds the dispersed carbon nanotubes to lithium manganese nickel oxide particles. Images by Evgenia Barannikova/UMBC.
“Biology provides several tools for us to solve important problems,†said Evgenia Barannikova, a graduate student at UMBC. “By mimicking biological processes we can find the better solution.â€
One of the inspirations for Barannikova’s research was the way that mollusks use peptides to control the growth of their shells. They demonstrate remarkable control in order to build intricate nano- and macrostructures from inorganic materials like calcium carbonate. The researchers borrowed the general approach of the mollusks, but had to get creative to find the appropriate peptide – no snail makes its shell from LMNO.
Barannikova and her colleagues used a procedure called phage display to screen more than a billion peptides in search of one that would stick strongly to LMNO. They isolated an appropriate peptide by combining a commercially produced library of amino acid sequences with a sample of the metal oxide, then repeatedly washing away the peptides that didn’t stick to it. They then combined the newly-discovered peptide with a previously isolated peptide that binds to carbon nanotubes.
The resulting peptide could form a bridge, binding to both the LMNO nanoparticles and the carbon nanotubes. By maintaining an organized architecture at the nanoscale, the researchers expect that their peptides will improve the power and cycling stability of Li-ion batteries, allowing them to be smaller and longer-lasting.
For her next act, Barannikova plans to make an anode with similar techniques and to integrate the two components. “I hope to demonstrate an entire biotemplated battery in my Ph.D. thesis,†she said.
Source: ChargedEVs



