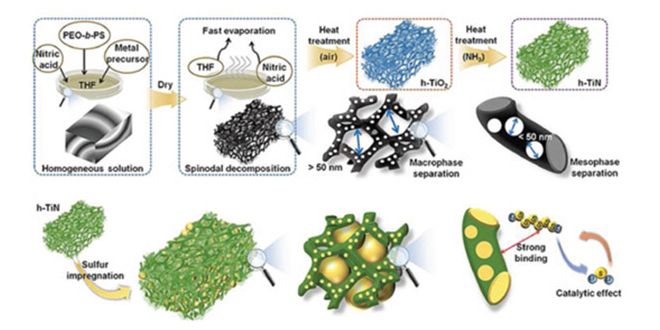
A research team from South Korean research university KAIST has developed stable, high-rate lithium-sulfur batteries (LSBs) by using hierarchically porous titanium nitride (h-TiN) as a sulfur host. In a paper in the journal Advanced Materials, the researchers report that h-TiN/S shows a reversible capacity of 557 mAh g-1 even after 1,000 cycles at 5 C rate with only 0.016% of capacity decay per cycle.
From the Advanced Materials paper:
“Lithium-sulfur batteries (LSBs) have garnered much attention as alternatives for existing lithium-ion batteries, owing to their high theoretical energy density of 2,600 Wh kg-1. In particular, sulfur, whose theoretical capacity is 1672 mAh g-1, is naturally abundant and environmentally benign. However, the practical use of LSB is still largely hindered by poor conductivity of sulfur, the dissolution of lithium polysulfides (LiPSs), and the sluggish redox kinetics.
“Hitherto, in contrast with other batteries, encapsulating sulfur into porous materials has been considered as a powerful strategy. Tailoring the porous architecture is a key for the further advance of LSBs because it plays crucial roles in determining LSB performance.
“Herein, we report the synthesis of co-continuous hierarchical macro- and mesoporous titanium nitride (h-TiN) as a multifunctional sulfur host. Metal nitrides are versatile materials in various research fields owing to their high electrical conductivity and excellent mechanical strength. Even though many researchers have attempted to control the nanostructure of metal nitrides, this work is the first to introduce a well-developed hierarchical macro- and mesoporous architecture on metal nitrides.”
To tackle those issues, KAIST Professor Jinwoo Lee and his team synthesized a hierarchical macro/mesoporous titanium nitride as a host material for sulfur.

Professor Jinwoo Lee, left, and PhD candidate Won-Gwang Lim
The titanium nitride has a high chemical affinity for sulfur and high electrical conductivity. As a result, it prevents the dissolution of active materials and facilitates the charge transfer. Moreover, the synergistic effect of macropore and mesopore structures allows the stable accommodation of large amounts of sulfur and facilitates the electrolyte penetration.
Previously reported polar inorganic materials have a high affinity for sulfur, but it was challenging to control the porous architecture suitable to the sulfur host. This work breaks such limitations by developing a synthetic route to easily control the porous architecture of inorganic materials, which led to obtaining superior cycle stability and high rate capabilities.
Source: ChargedEVs