The curious case of ionic liquids:Â Boulder Ionics/CoorsTek Specialty Chemicals Iolyte electrolytes
When you think of liquids under the brand name Coors, you think of the Silver Bullet, the Banquet Beer. You certainly don’t think of high technology, unless you’re really easily impressed by cold-activated cans. However, not everyone knows that there’s another huge conglomerate under the Coors name besides the $5 billion sudsmaker that was founded in 1873. The Coors family also lays claim to CoorsTek, Inc., a specialist in technical ceramics and other industrial products with 44 locations and more than $1 billion in annual sales that dates back to 1910.
Last October, CoorsTek acquired Boulder Ionics, a 2011 start-up that made the Cleantech Group’s 2014 Global Cleantech Ones to Watch list. Boulder Ionics’ novel lithium salts, ionic liquid electrolytes and other electrolytes made for energy storage devices had caught many others’ eyes as well. The company’s early and Series A funding came from the US Air Force and Navy, the National Science Foundation, Pangaea Ventures, CoorsTek’s investment branch and others. By the time Boulder started raising Series B capital, CoorsTek stepped in a full acquisition proposal.
“They said, ‘we want to commercialize your products, and we know about large-scale manufacturing,’†said Dr. Jerry L. Martin, co-founder and CEO of Boulder Ionics. “They have facilities all over the world and thousands of employees in the manufacturing space. We’re now part of an organization with global reach, a hundred years of manufacturing experience and the ability to rapidly move things from R&D into large-scale production.â€
With the acquisition, Martin will become President of the Boulder Ionics unit within the new CoorsTek Specialty Chemicals unit. Martin told us the Boulder Ionics name will probably dissolve in the next year. However, that won’t change what the company has become most known for – its ionic liquid electrolytes, branded as Iolytes, which promise improved safety, better performance at extreme temperatures, more power and longer life for the next-generation batteries and ultracapacitors that use them.
 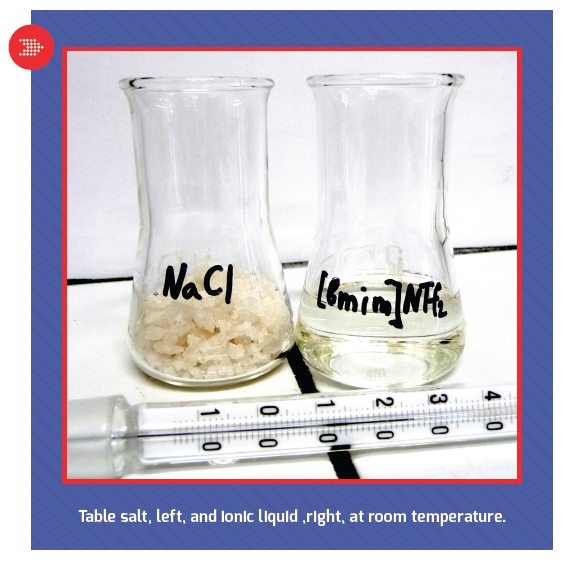
A slow flow
Every new battery materials technology seems to make the above claims, so what is it about ionic liquid electrolytes that makes them so special? For starters, an ionic liquid is a salt that’s molten at room temperature, which gives it some special properties in batteries.

“If that doesn’t make your head hurt, you weren’t listening in chemistry class,†Martin said, “because table salt melts at 800Ëš C. The idea of a salt that’s a molten liquid at room temperature is really crazy. This class of materials is relatively new; it was really developed in the mid-90s, mainly by some folks working at the Air Force. But once you figure out the secret to making low-temperature molten salts, there’s thousands and thousands of varieties to make.â€
With that many varieties, Martin cautions strongly against generalizing about ionic liquids; some will have this or that property and some will have the other, but few will have them all. “It’s like generalizing about metals,†he said. “Lead and platinum are very different.â€
Yet one can make a laundry list of possible advantages from Iolytes: high conductivity, low vapor pressure, non-flammability. Also, some ionic liquids are stable over very wide temperature ranges, especially very high temperatures – even above 250˚ C. Others have a melting point as low as -50˚ C.
“For example, you can dissolve sodium chloride (table salt) in water, and you’ll have water molecules, sodium ions and chlorine ions, but you have neutral water molecules,†Martin said. “In a molten salt, everything is an ion. There are no neutral molecules, and that leads to a set of unique properties, some quite interesting for batteries. Think about it: There’s nothing but ions in the mixture, so they can be highly conductive. Also, if you try to pull one of these ions out of the liquid, you have tremendous electrostatic forces pulling it back, so the net result of that is the vapor pressure of ionic liquids is essentially zero. They don’t boil; they don’t evaporate. You put a cup of ionic liquid on your desk, and come back 50 years later, it’s still going to be liquid. That lack of vapor pressure means that in general, they’re not flammable, because you can’t develop a flammable concentration that would burn above the surface.â€
Another great trait for many ionic liquids is greater electrochemical stability than the carbonate-based electrolytes common in current Li-ion batteries. With next-generation batteries often employing higher-voltage cathodes, they could exceed the safe limit of current electrolytes, which can lead to fires that can destroy life, property and the momentum of electrification.
“The current generation of electrolytes is based on highly flammable solvents that are marginally stable at voltages above about 4.2/4.3 V, which is where we operate our batteries today.†Some of Boulder’s ionic liquids can be stable at an absolute limit around 5.5 V, and even a lower practical limit should be plenty for next-generation batteries, which Martin said are looking at cathodes that operate between 4.6-5 V.
“People are trying all sorts of new things to replace components in the electrolyte with more stable materials,†Martin said. “One of those candidates is ionic liquids. If they let you raise the voltage, your energy is proportional to voltage; that’s why people are pursuing higher-voltage cathodes. But traditionally ionic liquids have been too expensive, not available in the required purity, and not available in industrial quantities.â€
The three Ps
Martin co-founded Boulder Ionics to work on the “three Ps†of ionic liquids: price, purity and production. All organic electrolytes need to be extremely pure.

“In some cases, you might have to limit chlorine to 10 ppm,†Martin said. “But most common synthesis techniques start with a chloride salt [laughs]. So you start with something that’s maybe 50% or 500,000 ppm, and you have to get that to 10 ppm. So over the past two decades, companies have figured out ways to do that on an industrial scale, but those techniques are not well developed for ionic liquids. A lot of our intellectual property – both patents and trade secrets – are on achieving those high purity levels cost-effectively and at scale.â€
While many companies are using a batch production process, Boulder Ionics gets more cost-effective results with a continuous production process. “We’re using what’s called micro-channel reactors and other process-intensification techniques to conduct these reactions very fast – in some cases, 100 times faster than typical practices. We can produce a very large amount of material from a relatively small system.â€
Martin drew an analogy of baking 10,000 cookies. You could either use an enormous oven to make thousands of cookies at a time, or come up with a system that made one cookie at a time at only a few seconds each.
“That’s really our approach,†he said. “Rather than building one big reactor that takes a long time to make these materials, we have a very small reactor that operates extremely fast and produces a large quantity of material. That allows us to build a modular system with modules capable of producing tens of tons of material per year, and they are the size of a refrigerator.â€
The company can purchase raw materials and use its system to put them through a series of chemical reactions for producing, purifying, drying and then packing the material straight into shipping vessels. “People come in expecting to see something like a refinery, and instead they see something that looks like racks of refrigerator-size modules all operating under computer control on a continuous basis,†Martin said.
While Boulder Ionics is not quite in full-scale production of its Iolytes yet, the company has several customers that are field-testing products using their materials. These early customers have to understand that the other P, price, will at first be higher for Boulder Ionics products than for the materials they are replacing. However, Martin is confident that his ionic liquids will reduce the cost of the final installed battery pack.
“We see our materials as enabling battery packs to be smaller, lighter and less expensive,†he said. “If our materials let the battery operate at higher temperatures, it could be that more manufacturers could adopt less expensive air cooling. Those are the kinds of things that really affect the cost: reducing the complexity of the cooling system, reducing the need to heat the battery on very cold days.â€
Martin expects to have industrial-scale production underway in late 2015 or early 2016. The company already has the production capabilities built for its Iolytes, and is building out production for its other breakthrough product, the Lithium FSI (fluorosulfonylimide) salt, aka LiFSI.
FSI: Boulder
CoorsTek Specialty Chemicals/Boulder Ionics has a hand in making all three electrolyte components: solvents, salts and additives. Its Iolytes can be either the solvent or the salt, depending on the end product. The company also produces other novel lithium salts, the marquee one being Lithium FSI. Boulder positions LiFSI as a good substitute for lithium PF6(hexafluorophosphate).

“Today, 90% of Li-ion batteries use the LiPF6 salt,†Martin said. “LiPF6 is one of those things that is not particularly good at anything, but it’s not particularly bad at anything. It’s the compromise that everybody has settled on, but it has some real limitations. One of those is, it isn’t very stable in the presence of water or at high temperatures. So one of the biggest causes of degradation to Li-ion batteries at elevated temperatures is the breakdown of the lithium salt. It goes through a series of reactions, and it eventually forms hydrofluoric acid, which of course is very corrosive.â€
In LiFSI, Martin thinks he has a leading candidate for the lithium salt that will be non-toxic, cost-effective and more stable than LiPF6, something researchers have sought for decades. He says it’s more conductive and more stable than LiPF6, giving batteries longer lives at wider temperature ranges. What’s also nice is that manufacturers can pretty easily swap out LiPF6 for LiFSI either in whole or in part.

“You obviously have to verify that it’s going to be compatible with all the other materials in your battery,†Martin said, “but in general our customers have not encountered difficulties in replacing it. And it doesn’t have to be a wholesale replacement; you could mix some of the salt you’re using now with Lithium FSI if you wanted to test the waters and still get some of the benefit. The challenges with LiFSI are the same: price, purity and production. It’s difficult to make very pure right now: it’s extraordinarily expensive, and there are no large-scale manufacturers anywhere in the world. However, some large battery companies are very seriously considering adopting it. Because of that, there’s a sort of global race on to figure out how to produce this material at a price and purity that meets market needs.â€
Martin feels that the recent acquisition by CoorsTek Special Chemicals positions the company well to win that race, because it will help in building the large-scale production to go along with Boulder Ionics’ patents in LiFSI synthesis.
I see Iolytes
When Boulder Ionics’ Iolytes go commercial, we could see them in consumer electronics, grid-scale energy storage, satellites, and certainly electric vehicles. The question is whether there are chemistries or formats to which they’ll be more suited. Martin said the Iolytes are stable with all the battery chemistries being seriously considered for next-generation high-voltage cathode systems. However, they do seem to be particularly appropriate for batteries using silicon anodes.
“Silicon can store much more lithium per unit volume than the carbon we use today,†Martin said, “but there’s always an interaction between the electrolyte and the anode that forms an SEI (solid electrolyte interface). It forms in the first couple of cycles, and if it’s stable, it prevents further degradation of the electrolyte. Well, the SEI on silicon is very different from the SEI on carbon. Right now, cycle life of those batteries is not good enough for automotive use. They can store a whole lot of energy, but then the battery degrades really fast. Recent work has shown that our materials form more stable SEI layers on silicon, allowing batteries with silicon anodes to last longer, in some cases by factors of two.â€
Martin also thinks that Iolytes may show up in the market first within ultracapacitors and Li-ion capacitors. Such capacitors are advancing at a much faster rate than batteries, Martin said, and they are essential to the start-stop technology used in the micro-hybrids that are widespread in Europe and beginning to creep into the US on models such as the Mazda 6.
“Over the next five years I think the United States will reach where Europe is now – where about half of the cars have some sort of start-stop energy recovery,†Martin said. “It’s the low-hanging fruit to meet the corporate average fuel economy (CAFE) standards.â€
The US armed forces backed Boulder Ionics early on, because the Air Force wanted to use ultracapacitors in satellites in low-Earth orbit, which undergo a charge/discharge cycle every 90 minutes or so. That adds up to tens of thousands of cycles over their lifetime, which traditional batteries can’t handle. To get around that, they would greatly oversize the battery and then only discharge it by a small amount. However, ultracapacitors can easily achieve a life of 100,000 cycles, making them much more efficient for satellites. Martin then reiterated one of the core benefits of the Iolytes: they would allow the ultracapacitors to operate at higher voltages, making them competitive with batteries.
“For many years, electrolytes were sort of ignored because they were good enough,†Martin said. “Now as everyone has been pushing on the voltage and asking for more safety, the industry’s realized that better electrolytes are key to next-generation batteries and ultracaps. There’s been a resurgence in development. It’s definitely an exciting time for the electrolyte industry.â€
Source: ChargedEVs



