Researchers at the Paul Scherrer Institute and ETH Zurich in Switzerland have developed a simple procedure that they say can enhance the performance of Li-ion batteries by improving the design of the electrodes without changing the underlying chemistry.
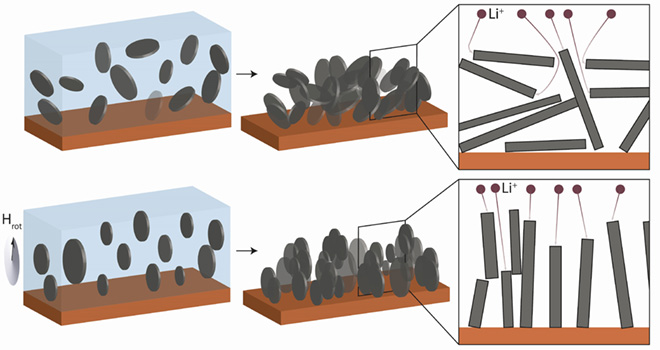
In “Magnetically aligned graphite electrodes for high rate performance Li-ion batteries,†published in Nature Energy, Juliette Billaud and colleagues explain that a major limitation of existing batteries has to do with the crooked, or “tortuous†path that ions must follow in highly loaded electrodes.
“The diffusion of charge carriers across thick graphite electrodes is often reduced by the high tortuosity of the porous anode structure, particularly when anisotropic flake-like particles are used,†write the Swiss team. “Aligning graphite flakes perpendicularly to the current collector could ease the transport of the charge carriers within the anode by creating short diffusion paths and exposing preferential insertion/extraction sites.â€
The researchers propose “a simple, up-scalable and inexpensive technique to orient graphite particles perpendicularly to the current collector by applying a low magnetic field during the electrode fabrication,†an approach that they have not seen reported before.
By optimizing the graphite anode on a conventional Li-ion battery, the researchers were able to enhance storage capacity by a factor of up to 3, under lab conditions. In a commercial battery pack, they estimate that this could translate to an improvement of 30 to 50 percent. The concept doesn’t call for a new battery design or new materials, but relies on improving existing components.
“We already have everything we need,†said Claire Villevieille, head of the Battery Materials Research Group at the Paul Scherrer Institute. “If a manufacturer were willing to take on production, enhanced batteries could be ready for the market within one or two years. The procedure is simple, cost-effective and scalable for use on rechargeable batteries in all areas of application, from wristwatch to smartphone, from laptop to car. And it has the additional bonus of being transferable to other anode-cathode batteries such as those based on sodium.â€

Juliette Billaud, co-first author of the new study, and Claire Villevieille, head of the battery materials research group at the Paul Scherrer Institute. (Photo: Markus Fischer/Paul Scherrer Institute)
Source: ChargedEVs