Although the material in a battery electrode may look uniform to the naked eye, at the atomic level, it’s a diverse landscape. Tiny variations in materials can affect reaction rates, and thus battery performance, in complex ways.
Professor Jiangyu Li and his colleagues at the University of Washington have built a new tool that could provide a better understanding of how chemical reactions progress at the level of atoms and molecules, creating “new opportunities to engineer materials properties so as to achieve quantum leaps in performance.â€
In “Scanning Thermo-ionic Microscopy for Probing Local Electrochemistry at the Nanoscale,†published in the Journal of Applied Physics, Jiangyu Li and his team describe a nanoscale probe that they developed. The concept is similar to atomic force microscopy – a tiny heated cantilever causes fluctuations in temperature and stress in the material beneath the probe. As the material expands and contracts, the cantilever vibrates in a way that can be measured using a laser beam, yielding a nanometer-scale map of the material.
The new approach has advantages over other types of atomic microscopy, and the team believes it will offer researchers a valuable tool for studying electrochemical material properties at the nanoscale. They tested it by measuring the concentration of charged species in Sm-doped ceria and LiFePO4, important materials in solid oxide fuel cells and lithium batteries.
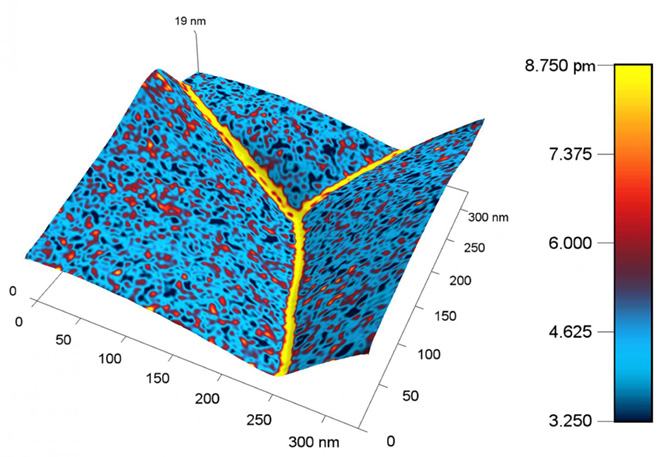
A nanoscale map of the metal ceria produced with the new probe shows a higher response, represented by a yellow color, near the boundary between grains of metal. The higher response corresponds to a higher concentration of charged species.
Image credit: Ehsan Nasr Esfahani/University of Washington
“The concentration of ionic and electronic species are often tied to important rate properties of electrochemical materials – such as surface reactions, interfacial charge transfer, and bulk and surface diffusion – that govern the device performance,†Li said. “By measuring these properties locally on the nanoscale, we can build a much better understanding of how electrochemical systems really work, and thus how to develop new materials with much higher performance.â€
Source: ChargedEVs