Metal-air battery technology could offer very high energy density, and is considered a promising candidate for “beyond Li-ion†next-generation batteries. Aluminum, which is abundant and cheap, is a likely anode material. Aluminum-air batteries have a theoretical specific energy of up to 8.1 kWh/kg, second only to lithium-air batteries (13.0 kWh/kg).
A pioneer in the aluminum-air field is the Israeli startup Phinergy, which formed a joint development agreement with aluminum giant Alcoa last February.
Now, Fuji Pigment says it has developed a new type of aluminum-air battery that can be recharged by refilling with salt or fresh water, and that it plans to commercialize the product by spring 2015. Dr. Ryohei Mori developed the technology, and described it in a paper in theJournal of the Electrochemical Society.
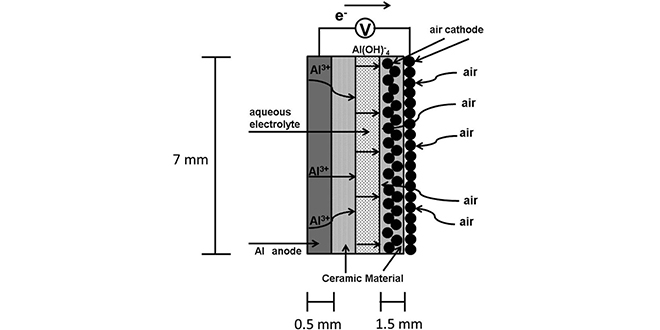
One problem with aluminum-air technology is parasitic hydrogen evolution caused by anode corrosion during discharge. To address this, Dr. Mori modified the battery structure by placing ceramic and carbonaceous materials between aqueous electrolyte and electrodes as an internal layer. This modified structure suppresses anode corrosion and by-product accumulation, resulting in longer battery life.
“In an earlier work, we demonstrated the use of ceramic aluminum ion conductors in preventing anodic corrosion while maintaining aluminum ion conductivity,†writes Dr. Mori. “We used Al2(WO4)3 as an aluminum ion conductor for both the anode and the air cathode. An aluminum-air battery with a rechargeable battery was fabricated, and its properties were stable for one month. We named this cell an ALFA cell.â€
“During this study, we noticed that Al2(WO4)3 had an intrinsic electrical conductivity of nearly 4 × 10−6 S cm−1 at 600◦ C; however, its conductivity was not high at room temperature. We found that the liquid electrolyte had penetrated the solid electrolyte region (the space between the ceramic particles), reaching the electrode. It was thus considered possible to fabricate a rechargeable aluminum–air battery with an insulating material, such as aluminum oxide, in order to replace the solid electrolyte.
“In our previous study, aqueous NaCl was used as an electrolyte. In the present study, we used NaOH and KOH to investigate the functionality of the ALFA cell and to verify the validity of our assumptions. Another aspect considered was that the major drawback of a metal-air battery is evaporation of its liquid electrolyte. Therefore, to investigate the possibility of preventing liquid electrolyte evaporation in the ALFA cell, we added glycerin to aqueous NaCl solution.â€
Source: Journal of the Electrochemical Society via Green Car Congress
Image courtesy of Mori 2015