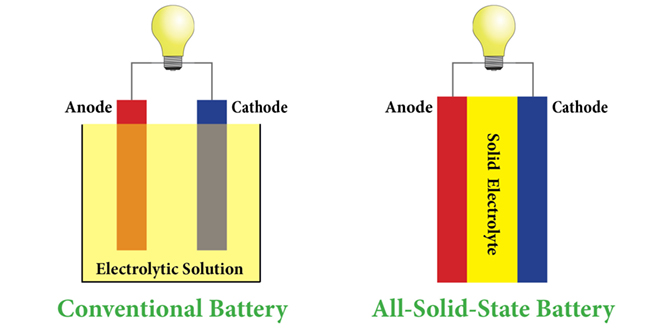
The latest advance in lithium-sulfur technology comes courtesy of a research team from Samsung and the University of Rome, who have built a solid-state Li-S battery with a capacity (∼1600 mAhg−1) approaching the theoretical value and Coulombic efficiency approaching 99%. In a paper published in the Journal of The Electrochemical Society, the team concludes that a solid electrolyte is “very effective in physically preventing polysulfide migration.†“Overcoming the polysulfide shuttle is a significant advantage, since it is a major drawback for a typical liquid electrolyte-based Li-S battery,†write Takanobu Yamada and colleagues. Li-sulfur batteries offer superior energy density, but there are still challenges to overcome, including the redox shuttle of polysulfides originating from the dissolution of the sulfur cathode material into the organic electrolyte, as well as poor lithium cycle performance due to the consumption of lithium metal during cycling. “Solid-state batteries have the favorable characteristic of avoiding lithium dendrite deposition and hence of preventing short circuits,†write the researchers. “In a previous work, we demonstrated a solid-state Li-S battery based on 0.8Li2S-0.2P2S5 electrolyte. However, even cycling under shallow depth of discharge, dendrite short circuits were observed. In this work we have extended the investigation by developing a battery using a stoichiometric composition of 0.75Li2S-0.25P2S5, Li3PS4 as the electrolyte.†In electrochemical testing, the Li/Li3PS4/S solid-state cell achieved the high specific capacity at both 25˚ C and 80˚ C. The team concluded that the high value of the Coulombic efficiency was clear evidence that polysulfide shuttle was prevented by the solid electrolyte layer. “Another important remark is that the discharge plateau typically reported for Li-S batteries was not seen at 25˚ C, where a large discharge-charge polarization was also observed. The electrochemical reaction was significantly accelerated at 80˚ C, where the cycling nearly evolved along the expected plateau. Although the reaction in the solid electrolyte Li-S cell is still unclear, its kinetics are expected to be much slower in the liquid electrolyte cell. “Realistically, the risk of dendrite formation and of cathode and electrolyte degradation cannot be excluded, and they might shorten the cycle life of the battery. Long cycle life and high Coulombic efficiency have been reported for thin-film lithium batteries where the electrolyte layers are generally prepared by a vapor deposition process and are quite dense. This suggests that a dense solid electrolyte is a key for making a high-performance solid-state battery.
Source: ChargedEVs