It’s a solar cell! No… it’s a rechargeable lithium-air battery! No… wait… it’s both: It’s the world’s first all-in-one solar battery!
The new device, developed by Ohio State University, is essentially an air-breathing lithium battery that recharges via a built-in solar cell. This is significant, because one of the biggest problems with wide-scale solar power deployment is that you also need huge banks of batteries to store electricity — to even out spikes in generation when it’s cloudy or dark — and not only are those batteries expensive, but a lot of electricity is lost simply by traveling from the solar panels to external storage. An integrated solution is both cheaper and more efficient — about 25% cheaper and 20% more efficient, according to the researchers.
Ohio State’s solar battery has an ingenious design. There are three electrodes: The standard lithium metal anode, an oxygen/air electrode in the middle, and a photoelectrode — a photovoltaic solar cell — on top. An electrolyte sandwich ensures that electrons can travel smoothly between each electrode. When the battery is charging, the solar cell is connected to the lithium electrode — on discharging, the lithium electrode is connected to the oxygen.
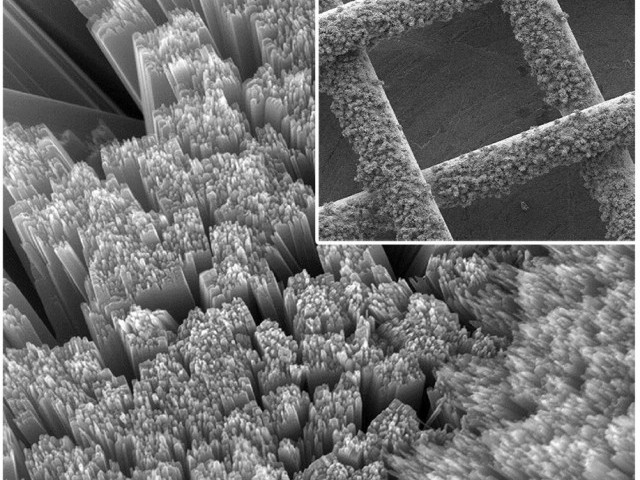
As such, there are two very distinct chemical reactions that occur inside the solar battery. When the battery discharges, the lithium reacts with the oxygen to create lithium peroxide (Li2O2) and electricity — in effect, it breathes in. When the battery recharges, the solar cell creates electrons (via the photovoltaic effect) that convert the lithium peroxide molecules back into lithium ions (Li+) and oxygen (O2) — i.e. the battery breathes back out. There’s an iodide “shuttle†in the electrolyte that helps ferry electrons between the electrodes. The solar cell itself is fairly simple and cheap dye-sensitized titanium dioxide panel, with iron oxide (rust) as the dye. Dye-sensitized solar cells (DSSCs) aren’t quite as efficient as silicon — they max out at around 15% at the moment — but they make up for it by being cheaper and much more rugged. (The picture at the top of the story is the titanium dioxide mesh.)
The battery — developed by Yiying Wu and friends at Ohio State — actually seems to be almost ready for production. The design is being patented. Wu says the design will be licensed to industrial partners, where it will help bring down the cost of solar power. “The state of the art is to use a solar panel to capture the light, and then use a cheap battery to store the energy,†Wu says. “We’ve integrated both functions into one device. Any time you can do that, you reduce cost†— in this case, according to Wu, by a massive 25%. [Research paper: doi:10.1038/ncomms6111 – “Integrating a redox-coupled dye-sensitized photoelectrode into a lithium–oxygen battery for photoassisted charging”]
The current design has pretty good characteristics: It produces plenty of power and energy, and the battery portion of the device has a lifetime comparable to existing rechargeable batteries. The research, which is funded by the US Department of Energy, will now look at enhancing the solar battery’s performance with new materials.
In practice, this solar battery could be a huge deal. In general, one of the biggest deal-breakers when it comes to mass adoption of renewable power generation is reliability: When the wind stops blowing or the sun goes behind a cloud, you need good ol’ fossil fuels (or nuclear if you’re lucky) to bridge the gap. Currently, the only real solution to this problem is pumping water back to the top of a hydroelectric dam (if there’s one nearby) — or using huge banks of batteries (so-called grid energy storage). Those banks of batteries aren’t cheap, and transferring and storing energy in them isn’t particularly efficient. Ohio State could be onto something big here.