Silicon anodes are receiving a great deal of attention, as they can enable batteries to deliver 3-5 times higher capacities compared with current graphite anodes.
Although silicon is abundant and cheap, Si anodes have a limited number of charge-discharge cycles – typically less than 100 times. Their volume expands enormously during each cycle, leading to fractures of the electrode particles or delamination of the electrode film.
A research team from the Korea Advanced Institute of Science & Technology (KAIST) reported a molecular pulley binder for high-capacity silicon anodes in the journal Science in July.
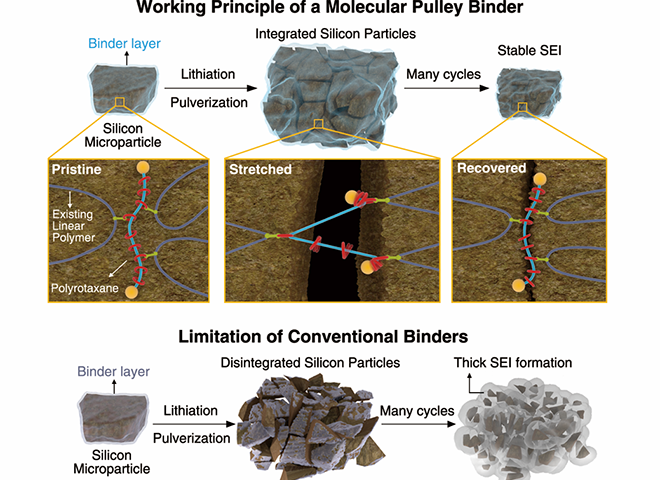
Professors Jang Wook Choi and Ali Coskun and colleagues integrated molecular pulleys, called polyrotaxanes, into a battery electrode binder, a polymer included in battery electrodes to attach the electrodes onto metallic substrates. In a polyrotaxane, rings are threaded into a polymer backbone, and can freely move along the backbone.
The free movement of the rings can follow the volume changes of the silicon particles. The rings’ sliding motion can hold Si particles without disintegration during their continuous volume change. Existing binders (usually simple linear polymers) with limited elasticity are not capable of holding pulverized particles firmly. Previous binders allowed pulverized particles to scatter, degrading the silicon electrode and reducing its capacity.
The authors said, “This is a good example of showing the importance of fundamental research. Polyrotaxane received a Nobel Prize last year, based on the concept called ‘mechanical bond.’ The ‘mechanical bond’ is a newly identified concept, and can be added to classical chemical bonds in chemistry, such as covalent, ionic, coordination, and metallic bonds. The long fundamental study is now expanding in an unexpected direction that addresses longstanding challenges in battery technology.”
The authors are currently collaborating with a major battery maker to get their molecular pulleys integrated into real battery products.
Source: ChargedEVs