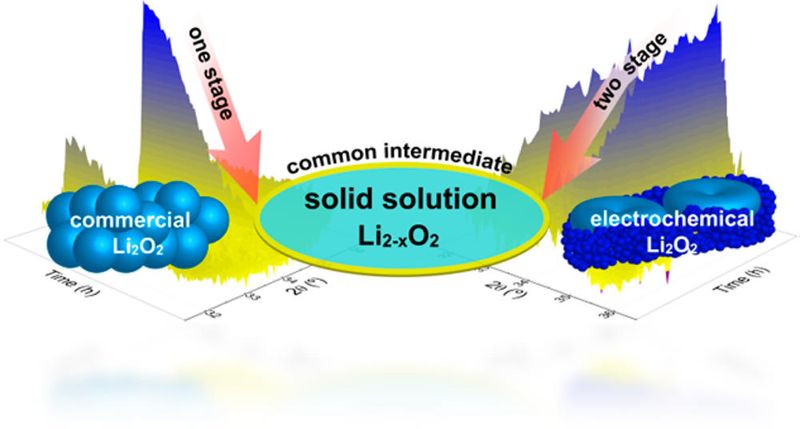
A team from Delft University in The Netherlands and the University of Waterloo in Canada has used operando X-ray diffraction to show that oxidation of electrochemically-generated Li2O2 in high energy density Li-air batteries occurs in two stages, but in only one step for commercial (crystalline) Li2O2. This discovery reveals a fundamental difference in the OER depending upon the nature of the peroxide.
In a paper published in the Journal of the American Chemical Society, the authors conclude that their findings not only reveal the fundamental nature of the charge reaction in Li−air batteries but also show the impact that the nature of the lithium peroxide (size, shape, and crystallinity) has on the oxidation mechanism. Controlling this process may be the key to high performance Li−air batteries, they suggest.
Research into nonaqueous Li−air/Li−O2 batteries has exploded over the past few years. This is primarily due to their high theoretical specific energy in the charged state (∼11500 Wh/kg), making them extremely attractive for electric automobiles. The electrochemical processes that drive this battery are represented by the total chemical reaction 2Li + O ⇄ Li2O2. Several bottlenecks that impede the functioning of this battery system need to be addressed before it can become viable.
These include the high (dis)charge overpotential resulting in a lower round trip efficiency, slow kinetics, electrolyte instability (side product formation) leading to poor cyclability, and the requirement of high purity O2. Of fundamental importance is the understanding of the mechanism of lithium peroxide [Li2O2] formation and oxidation and the governing factors. Over the past years there has been significant progress in the understanding of the Li2O2 formation process during discharge. Clear correlations have been established between the solvent donor number, discharge voltage, current density, and composition of the gas diffusion electrode on the morphology of Li2O2formed and the mechanism of their formation, be it via solution or on the electrode surface. But one of the many challenges of the Li−O2system includes the mechanism of Li2O2 oxidation which remains less well understood, with the elusive LiO2 superoxide intermediate remaining experimentally very difficult to observe.
…In this article, we combine operando X-ray diffraction (XRD), Rietveld refinement, calculations, and online electrochemical mass spectrometry (OEMS) to elucidate the mechanism of the oxidation evolution reaction, comparing electrochemically generated Li2O2 (E-Li2O2 ) and bulk crystalline (commercial) Li2O2 (C-Li2O2 ) during the charge reaction in a Li−O2 cell. A clear difference is observed between the oxidation of E-Li2O2 and C-Li2O2 which can be explained by the difference in the nature of the particles and crystallites. The OER mechanism, however, appears similar for both E-Li2O2 and C-Li2O2.
—Ganapathy et al.
The researchers fabricated Li-O2 cell gas diffusion electrodes (cathodes) by casting a mixture of activated carbon and a lithiated Nafion binder on carbon paper. They also fabricated preloaded Li2O2 cathodes combining Vulcan XC72 carbon, Li2O2 and PTFE; these were used in charge-only XRD experiments to probe the oxygen evolution mechanism.
The battery, comprising the cathode, a glass microfiber separator soaked with electrolyte, and a Li-metal anode, was assembled in the XRD cell.
They collected operando X-ray diffraction patterns on Li−O2 cells run for a complete (dis)charge cycle at low and intermediate current densities of 25 and 50 μA/cm2. They determined that smaller average crystallite sizes for E-Li2O2 are generated at a current density of 50 μA/cm2 and larger crystallite sizes are formed at 25 μA/cm2 at the end of discharge.
They observed different oxidation processes were observed for E-Li2O2 and C-Li2O2 associated with the differences in their nature.
- A two-stage oxidation for E-Li2O2 in which oxidation proceeds first through a noncrystalline (i.e., amorphous) lithium peroxide component, followed at higher potential by the crystalline peroxide via a Li-deficient solid solution (Li2–xO2) reaction.
They confirmed a platelet crystallite shape for the E-Li2O2, and speculated that the toroid particles are deconstructed one platelet at a time, starting with the smallest sizes that expose more peroxide surface.
- A single stage oxidation for bulk crystalline Li2O2 with an isotropic crystallite shape and larger crystallite dimensions. The observation of substoichiometric Li2−xO2 at the early stage of oxidation and the gradual decreasing average crystallite size suggests a small active fraction that also evolves oxygen via a Li deficient solid solution reaction. However, in this case the oxidation process gradually consumes the larger C-Li2O2crystallites.
The fundamental insight thus gained in the OER charge mechanism and its relation to the nature of the Li2O2 particles is essential for the design of future electrodes with lower overpotentials, one of the key challenges for high performance Li–air batteries.
—Ganapathy et al.
Resources
- Swapna Ganapathy, Brian D. Adams, Georgiana Stenou, Maria S. Anastasaki, Kees Goubitz, Xue-Fei Miao, Linda F. Nazar, and Marnix Wagemaker (2014) “Nature of Li2O2 Oxidation in a Li–O2 Battery Revealed by Operando X-ray Diffraction†Journal of the American Chemical Societydoi: 10.1021/ja508794r