Those doggone dendrites are the bane of battery boffins working on otherwise promising lithium metal anodes. Now a group of researchers from SLAC, Stanford and MIT have discovered that adding lithium polysulfide and lithium nitrate to an ether-based electrolyte can prevent dendrite growth and minimize electrolyte decomposition.
Professor Yi Cui, Professor Yet-Min Chiang (a co-founder of A123) and colleagues describe their work in “The Synergetic Effect of Lithium Polysulfide and Lithium Nitrate to Prevent Lithium Dendrite Growth,†which was recently published in Nature Communications.
The uncontrolled growth of dendrites is caused by the repeated stripping and plating of a lithium layer during cycling. Volume changes cause cracks in the solid-electrolyte interphase (SEI) that expose fresh lithium metal to the electrolyte, resulting in electrolyte decomposition.
“We demonstrate that the parasitic reaction between lithium polysulfide and lithium can instead be used to effectively suppress the growth of lithium dendrites,†wrote the team. “Both lithium polysulfide (Li2S8) and lithium nitrate (LiNO3) were used as additives to the ether-based electrolyte, which enables a synergetic effect leading to the formation of a stable and uniform SEI layer on the lithium surface that can greatly minimize the electrolyte decomposition and prevent dendrites from shooting out.â€
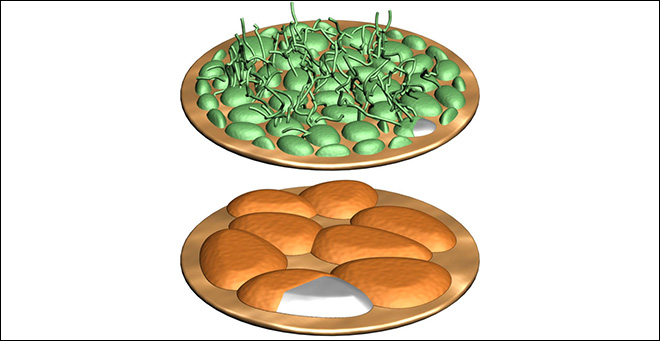
Deposits form on the anode of a lithium metal battery. When lithium nitrate is added to the electrolyte (top), destructive “fingers†of lithium metal, known as dendrites, grow on the surface. When lithium polysulfide is added as well (bottom), harmless pancake-like deposits form instead. (W. Li et al, Nature Communications)
The researchers assembled coin cells using various concentrations of the chemicals, and found that by manipulating the concentrations of Li2S8 and LiNO3, they could prevent the formation of lithium dendrites at a practical current density, and also demonstrated excellent cyclability.
Using in-situ optical imaging, the researchers observed that the polysulfide additive smooths the lithium dendrites through an etching effect.
“Our findings show that the reaction that has long been considered a critical flaw in lithium-sulfur batteries can actually benefit lithium metal-based battery systems when it is properly controlled,†they explain. “This illustrates a new strategy for solving dendrite issues associated with lithium metal anodes, which could be applied to next-generation high-energy-density battery systems such as lithium-sulfur and lithium-air batteries, as well as other metal-anode battery chemistries.â€
The next step is to see if this technique can prevent dendrite formation in larger cells that are closer to being practical batteries. It may also work for electrodes made of other metals, such as magnesium, calcium or aluminum.
Source: ChargedEVs


